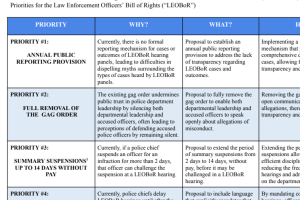
FDA Authorizes First Oral Antiviral for Treatment of COVID-19
Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for Pfizer’s Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kilograms or about[Read More…]













Comments